Inter Vs Intramolecular Bonds. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. This is the currently selected item. Hydrogen bonding is a form of attraction force between certain polar molecules. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. These bonds are very strong and take a lot of energy to break. When electrons are shared between two atoms, the bond is called a covalent bond. Think of these bonds as the bonds within. Ionic bonding is the attractive force between these oppositely charged ions. Intramolecular bonds form between atoms in a molecule. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Shenderovich 4,* and aleksander filarowski 1,* When one atom gives/ gain electron, the bond is called an ionic bond. Breaking this bonds results in the loss of identity of the molecule. Intramolecular forces determine chemical parameters of a substance.
Inter Vs Intramolecular Bonds , Ionic Bonding Is The Attractive Force Between These Oppositely Charged Ions.
Intermolecular Bonds Chemistry Socratic. When electrons are shared between two atoms, the bond is called a covalent bond. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Shenderovich 4,* and aleksander filarowski 1,* These bonds are very strong and take a lot of energy to break. When one atom gives/ gain electron, the bond is called an ionic bond. Hydrogen bonding is a form of attraction force between certain polar molecules. Ionic bonding is the attractive force between these oppositely charged ions. This is the currently selected item. Breaking this bonds results in the loss of identity of the molecule. Intramolecular forces determine chemical parameters of a substance. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Intramolecular bonds form between atoms in a molecule. Think of these bonds as the bonds within.
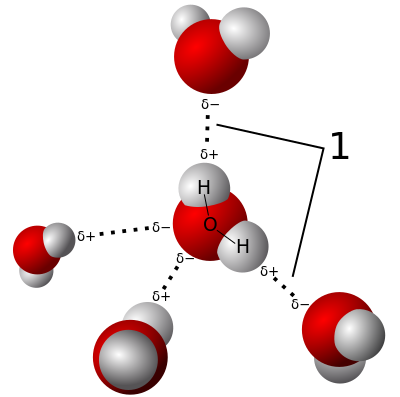
Generally intramolecular reactions are entropically favoured.
This is the currently selected item. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. 3) the lone pair(s) on f, o, or n increases the already partially negative charge on these atoms, The electrostatic interactions between the electrons and cations are called metallic bonding. The results have offered clear evidence for the existence of two different types of hydrogen‐bonding systems, inter‐ and intramolecular hydrogen bonds. Chemical bonds are the strong forces that bind atoms to make molecules and compounds. Here only ammonia and water have intermolecular hydrogen bonding.amout others i can only say that i have only learnt that methane and chloromethane molecules exist singularly. This attractive force can be intermolecular or intramolecular. The intermolecular forces are weaker and exists between molecules to hold the molecules together, while the intramolecular forces are stronger and exist within molecules to hold the atoms together. Ionic bonds are present in solid ionic crystals such as sodium chloride and in dissolved species such as grignard reagents (e.g., methylmagnesiumchloride dissol. Shenderovich 4,* and aleksander filarowski 1,* Take nacl (table salt) as an example. There are 3 types of intramolecular bonds: For example, the covalent bond, involving sharing electron pairs. Intramolecular forces intermolecular forces test question intermolecular forces the force of attraction or repulsion between molecules. H bonds are very polar. This video explains the difference between intramolecular forces and intermolecular forces and shows examples of both. Generally intramolecular reactions are entropically favoured. The journal of biological chemistry 0 1987 by the american society for biochemistry and molecular biology, he vol. And so that's different from an intramolecular force, which is the force within a molecule. Formed by reaction between different parts of the same molecule. The hydrogen bond structure and its influence on the amide‐containing benzoxazines phba‐a and ohba‐a have been studied by ft‐ir and 1 h nmr. Therefore, they are known as a sea of delocalized electrons. The evidence for the existence of these weak intermolecular forces is the fact that gases can be liquefied, that ordinary liquids exist and need a considerable input of energy for vaporization to a gas of independent molecules, and that many. 32, issue of november 15, pp. Hydrogen bonding is a form of attraction force between certain polar molecules. These bonds are generally stronger than the intermolecular bonds which exist between molecules. The subtle difference in the name comes from the latin roots of english with inter meaning between or among and intra meaning inside. Ionic bonding is the attractive force between these oppositely charged ions. This is the currently selected item. An intramolecular force is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules.
Help With Intermolecular Forces Organic Chemistry . Intramolecular Hydrogen Bond Patterns And Proton Dynamics In Nitrophthalic Acid Associates Kinga Jóźwiak 1, Aneta Jezierska 1, Jarosław J.
What Is The Difference Between Intermolecular And Intramolecular And What Is The Strongest Quora. Intramolecular forces determine chemical parameters of a substance. Breaking this bonds results in the loss of identity of the molecule. Intramolecular bonds form between atoms in a molecule. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Ionic bonding is the attractive force between these oppositely charged ions. When one atom gives/ gain electron, the bond is called an ionic bond. This is the currently selected item. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Think of these bonds as the bonds within. Shenderovich 4,* and aleksander filarowski 1,* When electrons are shared between two atoms, the bond is called a covalent bond. Hydrogen bonding is a form of attraction force between certain polar molecules. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. These bonds are very strong and take a lot of energy to break. Metals release electrons in their outer shells and these electrons are dispersed between metal cations.
Intermolecular Forces : Breaking This Bonds Results In The Loss Of Identity Of The Molecule.
Difference Between Intermolecular And Intramolecular Forces Definition Features Characteristics. This is the currently selected item. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. When electrons are shared between two atoms, the bond is called a covalent bond. Shenderovich 4,* and aleksander filarowski 1,* Ionic bonding is the attractive force between these oppositely charged ions. These bonds are very strong and take a lot of energy to break. When one atom gives/ gain electron, the bond is called an ionic bond. Think of these bonds as the bonds within.
Intramolecular And Intermolecular Forces Article Khan Academy . An intramolecular force is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules.
Intermolecular Forces. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Intramolecular forces determine chemical parameters of a substance. Breaking this bonds results in the loss of identity of the molecule. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Think of these bonds as the bonds within. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Intramolecular bonds form between atoms in a molecule. These bonds are very strong and take a lot of energy to break. When electrons are shared between two atoms, the bond is called a covalent bond. This is the currently selected item. Ionic bonding is the attractive force between these oppositely charged ions. Shenderovich 4,* and aleksander filarowski 1,* When one atom gives/ gain electron, the bond is called an ionic bond. Hydrogen bonding is a form of attraction force between certain polar molecules. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom.
Intermolecular And Interatomic Forces Intermolecular Forces Siyavula : Hello Everyone, Today I Will Be Getting The Basic Differences Between Intermolecular Forces, And Intramolecular Forces, And What They Influence, Etc.
Intermolecular Forces Ppt Video Online Download. These bonds are very strong and take a lot of energy to break. Intramolecular bonds form between atoms in a molecule. Breaking this bonds results in the loss of identity of the molecule. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Shenderovich 4,* and aleksander filarowski 1,* Intramolecular forces determine chemical parameters of a substance. Ionic bonding is the attractive force between these oppositely charged ions. When one atom gives/ gain electron, the bond is called an ionic bond. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Hydrogen bonding is a form of attraction force between certain polar molecules. This is the currently selected item. Think of these bonds as the bonds within. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. When electrons are shared between two atoms, the bond is called a covalent bond.
10 1 Intermolecular Forces General College Chemistry I . The Bond Is Held Together By The Force Of Attraction From The Opposite.
Intermolecular Bonds Chemistry Socratic. When one atom gives/ gain electron, the bond is called an ionic bond. Shenderovich 4,* and aleksander filarowski 1,* These bonds are very strong and take a lot of energy to break. When electrons are shared between two atoms, the bond is called a covalent bond. Breaking this bonds results in the loss of identity of the molecule. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Hydrogen bonding is a form of attraction force between certain polar molecules. Ionic bonding is the attractive force between these oppositely charged ions. Intramolecular bonds form between atoms in a molecule. This is the currently selected item. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Think of these bonds as the bonds within. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Intramolecular forces determine chemical parameters of a substance.
The Four Intermolecular Forces And How They Affect Boiling Points : Intermolecular Forces (Imf) Are The Forces Which Mediate Interaction Between Atoms, Including Forces Of Attraction Or Repulsion Which Act Between Atoms And Other Types Of Neighboring Particles, E.g.
Prelim Chemistry Intermolecular And Intramolecular Bonding Art Of Smart. Hydrogen bonding is a form of attraction force between certain polar molecules. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. These bonds are very strong and take a lot of energy to break. Ionic bonding is the attractive force between these oppositely charged ions. Breaking this bonds results in the loss of identity of the molecule. Intramolecular bonds form between atoms in a molecule. When electrons are shared between two atoms, the bond is called a covalent bond. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Think of these bonds as the bonds within. When one atom gives/ gain electron, the bond is called an ionic bond. This is the currently selected item. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Intramolecular forces determine chemical parameters of a substance. Shenderovich 4,* and aleksander filarowski 1,*
Intramolecular And Intermolecular Forces Article Khan Academy : These Bonds Are Generally Stronger Than The Intermolecular Bonds Which Exist Between Molecules.
Chemistry Liquids And Solids 2 Of 59 Intermolecular And Intramolecular Forces Youtube. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Ionic bonding is the attractive force between these oppositely charged ions. Shenderovich 4,* and aleksander filarowski 1,* When electrons are shared between two atoms, the bond is called a covalent bond. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. Breaking this bonds results in the loss of identity of the molecule. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. These bonds are very strong and take a lot of energy to break. This is the currently selected item. Hydrogen bonding is a form of attraction force between certain polar molecules. When one atom gives/ gain electron, the bond is called an ionic bond. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Intramolecular forces determine chemical parameters of a substance. Intramolecular bonds form between atoms in a molecule. Think of these bonds as the bonds within.
Intermolecular And Interatomic Forces Intermolecular Forces Siyavula : There Are 3 Types Of Intramolecular Bonds:
Figure 8 From Competing Intramolecular Vs Intermolecular Hydrogen Bonds In Solution Semantic Scholar. This is the currently selected item. Breaking this bonds results in the loss of identity of the molecule. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. Shenderovich 4,* and aleksander filarowski 1,* Hydrogen bonding is a form of attraction force between certain polar molecules. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. When electrons are shared between two atoms, the bond is called a covalent bond. Intramolecular bonds form between atoms in a molecule. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. When one atom gives/ gain electron, the bond is called an ionic bond. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Think of these bonds as the bonds within. These bonds are very strong and take a lot of energy to break. Ionic bonding is the attractive force between these oppositely charged ions. Intramolecular forces determine chemical parameters of a substance.
Ak Lectures Intramolecular And Intermolecular Forces : 32, Issue Of November 15, Pp.
Intramolecular And Intermolecular Forces Article Khan Academy. Hydrogen bonding is a form of attraction force between certain polar molecules. This is the currently selected item. Intramolecular forces determine chemical parameters of a substance. When electrons are shared between two atoms, the bond is called a covalent bond. Ionic bonding is the attractive force between these oppositely charged ions. Shenderovich 4,* and aleksander filarowski 1,* Intramolecular bonds form between atoms in a molecule. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. When one atom gives/ gain electron, the bond is called an ionic bond. Metals release electrons in their outer shells and these electrons are dispersed between metal cations. Breaking this bonds results in the loss of identity of the molecule. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. Think of these bonds as the bonds within. These bonds are very strong and take a lot of energy to break. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together.
Intramolecular And Intermolecular Forces Article Khan Academy . Intramolecular Hydrogen Bond Patterns And Proton Dynamics In Nitrophthalic Acid Associates Kinga Jóźwiak 1, Aneta Jezierska 1, Jarosław J.
Intermolecular Forces. Intramolecular forces determine chemical parameters of a substance. Breaking this bonds results in the loss of identity of the molecule. Intramolecular bonds form between atoms in a molecule. Intramolecular hydrogen bond patterns and proton dynamics in nitrophthalic acid associates kinga jóźwiak 1, aneta jezierska 1, jarosław j. This is the currently selected item. Ionic bonding is the attractive force between these oppositely charged ions. Intramolecular interactions occur when two atoms share electrons or donate/gain electrons to/from another atom. When one atom gives/ gain electron, the bond is called an ionic bond. Hydrogen bonding is a form of attraction force between certain polar molecules. Think of these bonds as the bonds within. Shenderovich 4,* and aleksander filarowski 1,* Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bonds.there are two types of attractive forces that keep the covalent molecules together. These bonds are very strong and take a lot of energy to break. When electrons are shared between two atoms, the bond is called a covalent bond. Metals release electrons in their outer shells and these electrons are dispersed between metal cations.